Valence Electrons & Bohr DiagramsPage
1
1
Slide 1
Valence Electrons & Bohr Diagrams
Slide 2
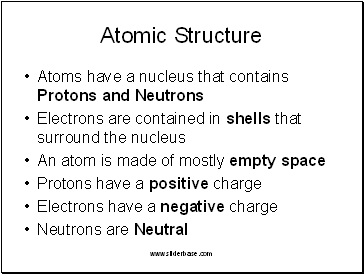
Atomic Structure
Atoms have a nucleus that contains Protons and Neutrons
Electrons are contained in shells that surround the nucleus
An atom is made of mostly empty space
Protons have a positive charge
Electrons have a negative charge
Neutrons are Neutral
Slide 3
Valence Electrons
Each electron shell can hold a certain number of electrons
Electron shells are filled from the inside out
Noble Gases have full outer electron shells
All other elements have partially filled outer electron shells
Slide 4
Valence Electrons
The electrons in the outer most electron shell are called valence electrons
The shell containing electrons that is furthest from the nucleus is called the valence shell
The number of electron shells with electrons is the same as the period number
Slide 5
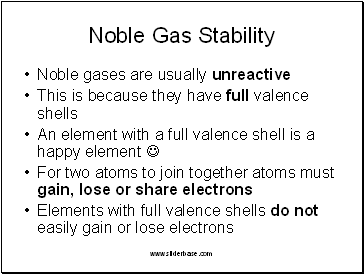
Noble Gas Stability
Noble gases are usually unreactive
This is because they have full valence shells
An element with a full valence shell is a happy element
For two atoms to join together atoms must gain, lose or share electrons
Elements with full valence shells do not easily gain or lose electrons
Slide 6
Noble Gas Stability
Atoms want to gain stability
Atoms will try to gain or lose electrons to have a full valence shell
Metals try to lose electrons
Non-Metals try to gain electrons
Slide 7
Becoming An Ion
Electrons are negatively charged
Protons are positively charged
Neutral atoms do not have a charge because the number of protons is the same as the number of electrons
When atoms gain or lose electrons they become positively or negatively charged
An atom with a charge is called an Ion
Slide 8
Bohr Models
Niels Bohr created a visual model of the atom to make them easy to understand
A Bohr Model contains a central nucleus surrounded by electron shells
For each model you state the number of protons and neutrons in the nucleus and draw a dot on the electron shells for each electron
Contents
Last added presentations
- Sound
- Space Radiation
- History of Modern Astronomy
- Resource Acquisition and Transport in Vascular Plants
- Simulation at NASA for the Space Radiation Effort
- Newton’s Law of Gravity
- Solar Energy