Chemical and NuclearPage
1
1
Slide 1
Balancing Equations:
Chemical and Nuclear
Slide 2
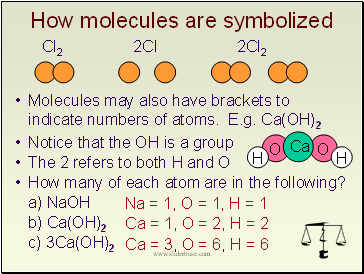
How molecules are symbolized
Cl2 2Cl 2Cl2
Molecules may also have brackets to indicate numbers of atoms. E.g. Ca(OH)2
Notice that the OH is a group
The 2 refers to both H and O
How many of each atom are in the following?
a) NaOH
b) Ca(OH)2
c) 3Ca(OH)2
Na = 1, O = 1, H = 1
Ca = 1, O = 2, H = 2
Ca = 3, O = 6, H = 6
Slide 3
Balancing equations: MgO
The law of conservation of mass states that matter can neither be created or destroyed
Thus, atoms are neither created or destroyed, only rearranged in a chemical reaction
Thus, the number of a particular atom is the same on both sides of a chemical equation
Example: Magnesium + Oxygen (from lab)
Mg + O2 MgO
However, this is not balanced
Left: Mg = 1, O = 2
Right: Mg = 1, O = 1
Slide 4
Balance equations by “inspection”
Hints: start with elements that occur in one compound on each side. Treat polyatomic ions that repeat as if they were a single entity.
5
2
3
3.5
2
7
4
6
2
2
2
2
6
3
C2H6 + O2 CO2 + H2O
a) P4 + O2 P4O10
b) Li + H2O H2 + LiOH
c) Bi(NO3)3 + K2S Bi2S3 + KNO3
d) C2H6 + O2 CO2 + H2O
From Mg + O2 MgO
2Mg + O2 2MgO is correct
Mg + ½O2 MgO is incorrect
Mg2 + O2 2MgO is incorrect
4Mg + 2 O2 4MgO is incorrect
Slide 5
Balance these skeleton equations:
a) Mg + 2HCl MgCl2 + H2
b) 3Ca + N2 Ca3N2
c) NH4NO3 N2O + 2H2O
d) 2BiCl3 + 3H2S Bi2S3 + 6HCl
e) 2C4H10 + 13O2 8CO2 + 10H2O
f) 6O2 + C6H12O6 6CO2 + 6H2O
g) 3NO2 + H2O 2HNO3 + NO
h) Cr2(SO4)3+ 6NaOH 2Cr(OH)3+ 3Na2SO4
i) Al4C3 + 12H2O 3CH4 + 4Al(OH)3
Slide 6
Returning to reaction types
We have looked at several types of reactions without worrying about balancing
However, all equations should be balanced
Predict the products and balance these:
(recall, metals above replace metals below, reactions with water yield metal hydroxides)
Cu + Fe2(SO4)3
NR (no reaction)
Zn + Li2CO3
Cu + AlCl3
Fe + CuSO4
LiOH + H2
Al2O3
2
Ni + NaCl
Al + CuCl2
1 2
Contents
- How molecules are symbolized
- Balancing equations: MgO
- Balance equations by “inspection”
- Balance these skeleton equations:
- Returning to reaction types
- Discovery of Radioactivity
- Types of Radioactivity
- Nuclear equations
Last added presentations
- Motion
- Magnetic field uses sound waves to ignite sun's ring of fire
- Simulation at NASA for the Space Radiation Effort
- Sound
- Mechanics Lecture
- Static and Kinetic Friction
- Buoyancy