C1a Rocks and MetalsPage
1
1
Slide 1
C1a Rocks and Metals
2.2 Extracting Iron
Slide 2
Learning objectives
Understand which metals can be extracted using carbon
Be able to describe how a blast furnace works
Slide 3
What do you understand?
How would you rate yourself when thinking about extraction of metals?
Slide 4
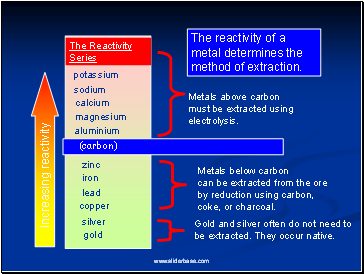
The Reactivity
Series
potassium
sodium
calcium
magnesium
aluminium
zinc
iron
copper
gold
(carbon)
Increasing reactivity
Metals above carbon must be extracted using electrolysis.
Metals below carbon
can be extracted from the ore by reduction using carbon, coke, or charcoal.
Gold and silver often do not need to
be extracted. They occur native.
The reactivity of a metal determines the method of extraction.
lead
silver
Slide 5
Iron age
Iron – 2nd most common metal in the Earth’s crust
Found as haematite
Iron (III) oxide (Fe2O3)
Sand
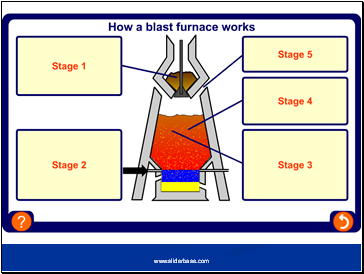
Can be obtained using a blast furnace
Slide 6
Slide 7
Reduction of iron ore
carbon + oxygen carbon dioxide
Carbon dioxide + carbon carbon monoxide
Carbon monoxide + iron oxide iron + carbon dioxide
Slide 8
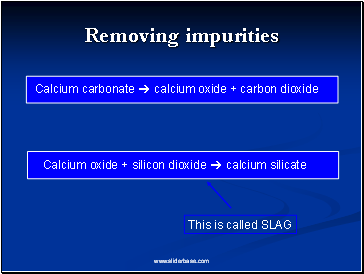
Removing impurities
Calcium carbonate calcium oxide + carbon dioxide
Calcium oxide + silicon dioxide calcium silicate
This is called SLAG
Slide 9
Summary of the process
Iron is not found naturally, but in Iron Ore
We can extract iron form iron ore using a Blast Furnace
Using chemistry we can produce pure iron
Limestone (CaCO3) is used to remove impurities
Coke/Carbon gives us the high temperature we need for the reactions to take place
Slide 10
Learning outcomes
Why can some metals be extracted using carbon when some can’t?
What do we use to extract iron?
How does a blast furnace work?
What chemical reactions take place?
Slide 11
Extraction of Iron
1 2
Contents
- C1a Rocks and Metals
- Iron age
- Reduction of iron ore
- Removing impurities
- Summary of the process
- Learning outcomes
Last added presentations
- Madame Marie Curie
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Simulation at NASA for the Space Radiation Effort
- Gravitation
- Newton’s Laws of Motion
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Heat-Energy on the Move