Arrhenius Acids and BasesPage
1
1
Slide 1

LecturePLUS Timberlake
1
Chapter 9 Acids and Bases
Acids and Bases
Slide 2

Arrhenius Acids and Bases
LecturePLUS Timberlake
2
Acids produce H+ in aqueous solutions
water
HCl H+(aq) + Cl- (aq)
Bases produce OH- in aqueous solutions
water
NaOH Na+(aq) + OH- (aq)
Slide 3
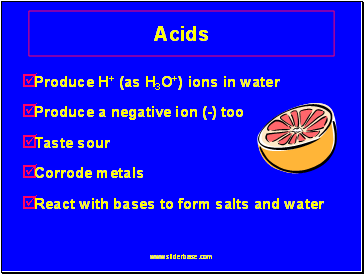
Acids
LecturePLUS Timberlake
3
Produce H+ (as H3O+) ions in water
Produce a negative ion (-) too
Taste sour
Corrode metals
React with bases to form salts and water
Slide 4
LecturePLUS Timberlake
4
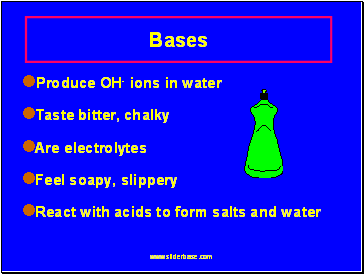
Bases
Produce OH- ions in water
Taste bitter, chalky
Are electrolytes
Feel soapy, slippery
React with acids to form salts and water
Slide 5

LecturePLUS Timberlake
5
Learning Check AB1
Describe the solution in each of the following as: 1) acid 2) base or 3)neutral.
A. _soda
B. _soap
C. _coffee
D. _ wine
E. _ water
F. _ grapefruit
Slide 6
LecturePLUS Timberlake
6
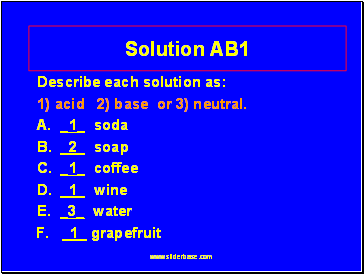
Solution AB1
Describe each solution as:
1) acid 2) base or 3) neutral.
A. _1_ soda
B. _2_ soap
C. _1_ coffee
D. _1_ wine
E. _3_ water
F. _1_ grapefruit
Slide 7

LecturePLUS Timberlake
7
Learning Check AB2
Identify each as characteristic of an A) acid or B) base
1. Sour taste
2. Produces OH- in aqueous solutions
3. Chalky taste
4. Is an electrolyte
5. Produces H+ in aqueous solutions
Slide 8

LecturePLUS Timberlake
8
Solution AB2
Identify each as a characteristic of an A) acid or B) base
_A_ 1. Sour taste
_B_ 2. Produces OH- in aqueous solutions
_B_ 3. Chalky taste
A, B 4. Is an electrolyte
_A_ 5. Produces H+ in aqueous solutions
Slide 9

LecturePLUS Timberlake
9
Some Common Acids
HCl hydrochloric acid
HNO3 nitric acid
H3PO4 phosphoric acid
H2SO4 sulfuric acid
CH3COOH acetic acid
Slide 10
LecturePLUS Timberlake
10
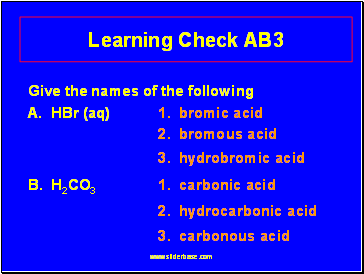
Learning Check AB3
Give the names of the following
1 2
Contents
Last added presentations
- Health Physics
- Mechanics Lecture
- Radiation
- Thermal Energy
- Heat-Energy on the Move
- Ch 9 Nuclear Radiation
- Resource Acquisition and Transport in Vascular Plants
