Valence ElectronsPage
1
1
Slide 1
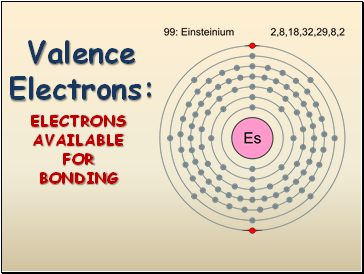
Valence Electrons:
ELECTRONS AVAILABLE FOR BONDING
Slide 2
CA Standards
Students know how to use the periodic table to determine the number of electrons available for bonding.
Students know how to draw Lewis dot structures.
Slide 3
Definition
Valence electrons are electrons in the outmost shell (energy level). They are the electrons available for bonding.
Slide 4
Group 1 (alkali metals) have 1 valence electron
Slide 5
Group 2 (alkaline earth metals) have 2 valence electrons
Slide 6
Group 13 elements have 3 valence electrons
Slide 7
Group 14 elements have 4 valence electrons
Slide 8
Group 15 elements have 5 valence electrons
Slide 9
Group 16 elements have 6 valence electrons
Slide 10
Group 17 (halogens) have 7 valence electrons
Slide 11
Group 18 (Noble gases) have 8 valence electrons, except helium, which has only 2
Slide 12
Transition metals (d block) have 1 or 2 valence electrons
Slide 13
Lanthanides and actinides
(f block) have 1 or 2 valence electrons
Slide 14
Dot Notations
An atoms valence electrons can be represented by Lewis dot notations.
Slide 15
Dot Notations Period 2
Lewis dot notations for the valence electrons of the elements of Period 2.
Contents
Last added presentations
- Solar Energy
- Radiation Safety and Operations
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- History of Modern Astronomy
- Madame Marie Curie
- Resource Acquisition and Transport in Vascular Plants
- The Effects of Radiation on Living Things