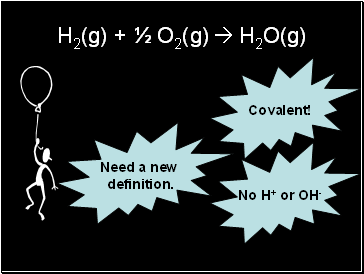Redox ReactionsPage
1
1
Slide 1
Redox Reactions.
Oxidation
Reduction
Slide 2
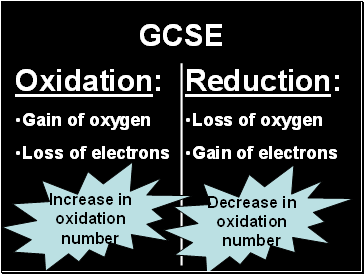
GCSE
Oxidation:
Gain of oxygen
Loss of electrons
Reduction:
Loss of oxygen
Gain of electrons
Increase in
oxidation
number
Decrease in
oxidation
number
Slide 3
4 Experiments:
Burning magnesium
Copper in silver nitrate solution
Chlorine solution and potassium iodide solution
Exploding hydrogen
Word equation
Balanced symbol equation
Slide 4
2Mg(s) + O2(g) 2MgO(s)
Oxidised – gains oxygen
Must be a redox!
Mg Mg2+
O O2-
Put the
e- in.
+2e-
+2e-
Oxidised – loss of e-
Reduced – gain of e-
Slide 5
Cu(s) + 2AgNO3(aq) Cu(NO3 )2(aq) + 2Ag(s)
Ag+ Ag
Cu Cu2+
Complete the half-equations
+e-
+2e-
Oxidised?
Reduced?
Oxidised – loss of e-
Reduced – gain of e-
Slide 6
Try Question 1.
Slide 7
H2(g) + ½ O2(g) H2O(g)
Covalent!
No H+ or OH-
Need a new
definition.
Slide 8
GCSE
Oxidation:
Gain of oxygen
Loss of electrons
Reduction:
Loss of oxygen
Gain of electrons
Increase in
oxidation
number
Decrease in
oxidation
number
Slide 9
Oxidation Numbers
The oxidation number of an atom in an element is zero. E.g. Mg in Mg, O in O2.
Slide 10
Oxidation Numbers
The oxidation numbers of atoms in a compound add up to zero.
Oxidation state of C in CO2?
? – 4 = 0
? = +4
Put the +!
Slide 11
Oxidation Numbers
The oxidation numbers of atoms in a compound add up to zero.
Oxidation state of Mg in MgCl2?
+2
Slide 12
Oxidation Numbers
The oxidation numbers of atoms in a compound add up to zero.
Oxidation state of N in NH3?
-3
Slide 13
1 2
Contents
Last added presentations
- Madame Marie Curie
- Thermal Energy
- Heat-Energy on the Move
- Direct heat utilization of geothermal energy
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Static and Kinetic Friction
- Waves & Sound